Diseases caused by fungal pathogens affect over a billion people across the globe and kill just under 2 million people each year; however, these estimates are understated because of a lack of mandatory public health surveillance of fungal diseases. The World Health Organization has issued “a call to action” stating that fatal fungal diseases threaten global health. The fungi, Aspergillus fumigatus, Candida albicans, Candida auris and Cryptococcus neoformans have been designated as threats of highest priority.
Neuroinfections: Resolving mechanisms of fungal dissemination to the central nervous system.
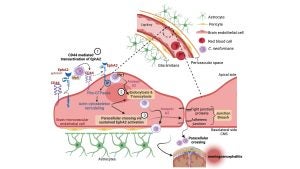
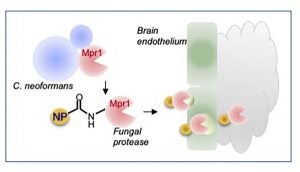
We study the pathogenesis of human fungal pathogens with a particular focus on fungi that disseminate to the central nervous system (CNS). Among the pathogens we study is Cryptococcus neoformans (Cn) – the leading cause of fungal meningoencephalitis. Our studies are aimed at resolving the molecular mechanisms mediating the interplay between fungi and the CNS. This interest was spearheaded in part by our study of the extracellular proteome of Cn, where we identified a key fungal secreted metalloprotease, Mpr1, that promotes fungal disease in the CNS. In parallel studies, we identified EphA2, a tyrosine kinase receptor in brain microvascular endothelial cells (a.k.a. the blood-brain barrier) and demonstrated that Cn engages EphA2 in order to access the CNS.
Blood-brain barrier organoids (spheroids) – an in vitro model of the blood-brain barrier.
We have adapted human BBB-organoids to investigate invasion of the CNS by Cn. These organoids are formed by combining immortalized brain endothelial cells (IBMECs) or primary human brain endothelial cells, primary astrocytes and primary pericytes in a 1:1:1 ratio in a low-adhesion 96-well plates that are specially designed to encourage the formation of unitary and uniformly sized (~200 µm) organoids . Scaffold support is not required for this process to occur indicating that each cell type is programmed for formation of the BBB architecture. A 3D model such as the one we have re-developed has never been applied to neuro-invasive studies of pathogens. The BBB organoids are produced routinely and each iteration recapitulates phenotypes associated with a functional BBB. BBB organoids are either examined in real time or they are fixed and sectioned for confocal studies. We have confirmed the integrity of the organoids by TEM and by measuring BBB permeability in these organoids, using fluorophore-conjugated to high molecular weight, BBB impermeable compounds (dextran, bovine serum albumin).
UC Davis CounterACT Center of Excellence – Identifying improved medical countermeasures for chemical threat agents that cause seizures in humans.
https://counteract.vetmed.ucdavis.edu/
https://counteract.vetmed.ucdavis.edu/projects/project-2
Project 2: Assess strategies for modulating blood-brain barrier (BBB) and neurovascular function following acute organophosphate intoxication.
Project 2 is testing the hypothesis that therapies that reverse BBB dysfunction when administered as adjuncts to standard of care will mitigate the long-term, adverse neurological consequences of acute intoxication from organophosphate (OP) nerve agents. Clinical and experimental evidence shows that current standard of care for acute OP intoxication (atropine, oxime, and benzodiazepine) does not sufficiently protect against persistent neuropathology or prevent development of spontaneous seizures and cognitive deficits in survivors. Project 2 is based on the fact that BBB is vital for normal brain health and BBB dysfunction is associated with epilepsy and the emergence of spontaneous seizures. It may be involved in cognitive impairment as well. With the use of a well established model of acute diisopropylflurophosphate intoxication (DFP is a surrogate for OP nerve agents that cause life-threatening seizures), Project 2 aims to:
- Characterize the spatiotemporal profile of BBB dysfunction following acute OP intoxication.
- Identify mechanisms contributing to OP-induced BBB/neurovascular dysfunction.
- Determine the efficacy of therapeutic candidates in spontaneous recurrent seizures and cognitive impairment following acute OP intoxication.
Translational Studies.
Current studies include translation of our basic research to identify novel peptides with antifungal activity, to develop novel antifungals via drug screens aimed at blocking Mpr1 protease activity, and to develop a platform technology that will deliver therapeutic drugs across the blood-brain barrier by conjugating Mpr1 to drug-loaded nanocarriers.
Discovery of novel of peptides with antifungal activity.
Despite the morbidity and mortality caused by fungal infections, and the looming threat of multi-resistance, there has been no substantial advancement in the development of antifungal agents. Although antifungal peptides (AFPs) are gaining popularity as potentially new antifungal agents, the lack of innovation in the AFP space has stifled development. To tackle these major challenges, we capitalized on the one-bead one-compound (OBOC) combinatorial technology (invented by Dr. Kit Lam, our collaborator) and devised a highly innovative high-throughput, multi-step, fluorescence-based platform for screening chemically synthesized peptide libraries to identify membrane-active antifungal peptides on the basis of their ability to discriminate between fungal and mammalian plasma membrane compositions of giant unilamellar vesicles. The proposed research will test the hypothesis that the enabling OBOC technology, in combination with powerful screening strategies will enable us to rapidly develop and optimize novel antifungal agents possessing defined physiochemical features required for broad-spectrum antifungal activity, diverse mechanisms of action and a high therapeutic index. The complementary expertise of the PIs (Dr. Gelli & Dr. Lam) brings a unique focus and strength to the proposed studies that will ensure a productive and successful collaboration.
Antifungal activity of amyloid beta as a driver of dementia and Alzheimer’s disease (AD) pathogenesis.
The goal of the proposed studies is to resolve large gaps in our understanding of how brain fungal burden/infection impacts AD pathogenesis. We will examine the role of amyloid beta precursor protein (APP) in a mouse model of fungal brain infection and evaluate whether the antifungal activity of amyloid beta (Ab) is intrinsic and/or whether Ab promotes antifungal immunity.
Completed Projects
How endothelial cell senescence impacts the integrity of the blood-brain barrier.
Our interest in the blood-brain barrier during pathological conditions has resulted in an NIH-funded collaborative study, with Drs. Lein and Knowlton, (UCD) aimed at understanding how vascular endothelial cell senescence during aging alters blood-brain barrier function and how that impacts the onset of dementia.
Resolving mechanisms of Coccidioides pathogenesis.
Our research efforts include collaborative studies with Dr. Thompson (UCD), aimed at the characterization of the extracellular proteome of the parasitic phase of Coccidioides, the cause of Valley Fever in the southwestern United States . The goals are to resolve the role of these secreted proteins in the pulmonary-to-CNS dissemination of Coccidioides and to establish a novel diagnostic tool that would differentiate a pulmonary cancer nodule from a fungal nodule.
Contact: Angie Gelli, acgelli@ucdavis.edu, University of California, Davis